Cleanroom Classification
The purpose of a cleanroom is to provide a clean working environment that is relatively free of contamination.
Common contamination includes dust particles, moisture, bacteria, fungus, human skin, cosmetics, perfumes, lint, fibers, and many other contaminants. These contaminants could be introduced into the environment by the air entering the environment, cleaning products or by personnel and process equipment used in the environment.
The level of contamination is more detrimental to some processes and measurements than to others and cleanrooms are designed and built according to the application requirements. The practices and processes used within the cleanroom also are predefined by the level of contamination allowed in the cleanroom. Understandably, the build and operation cost for a high specification cleanroom with low contamination limits is much higher than the cost for a lower contamination limit cleanroom.
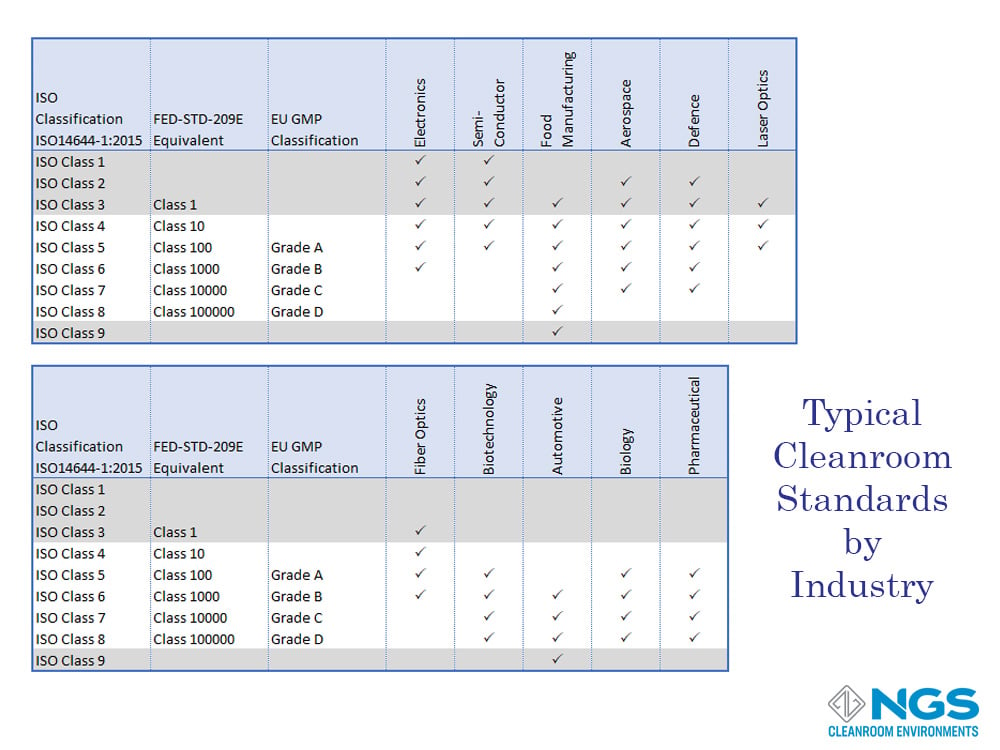
The application performed in the cleanroom whether it is production or measurement determines the level of acceptable (or allowed) contamination. These levels vary according to the industry and the product or measurement accuracy required. Industries where levels of contamination to the process are vital include medical, pharmaceutical, biotechnology, optics, aerospace, automotive, electronics, semiconductor, and food production.
Standards
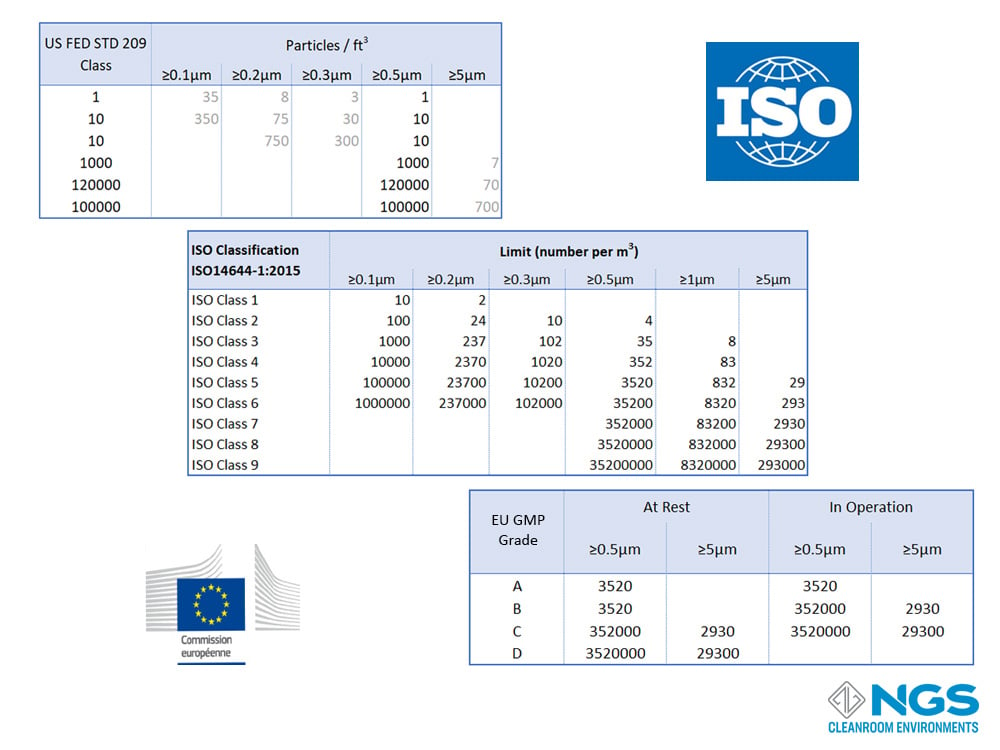
Internationally agreed standards were needed to classify the cleanroom environment in order to define a common language, to monitor contamination levels and to introduce best practices into cleanrooms. These standards were to classify the cleanroom environment according to the allowed contamination levels and the processes (practices) to follow in the cleanroom. The main classification standards for cleanrooms are ISO14644-1:2015, US FED STD 209E and GMP Annex European Community Cleanroom Standards. The level of contamination in each standard is based on the number of particles in a defined particle size range that is allowed in the air.
Cleanrooms are classified according to the cleanliness of the air in the room with the various cleanroom standards providing the identification.
Federal Standard 209
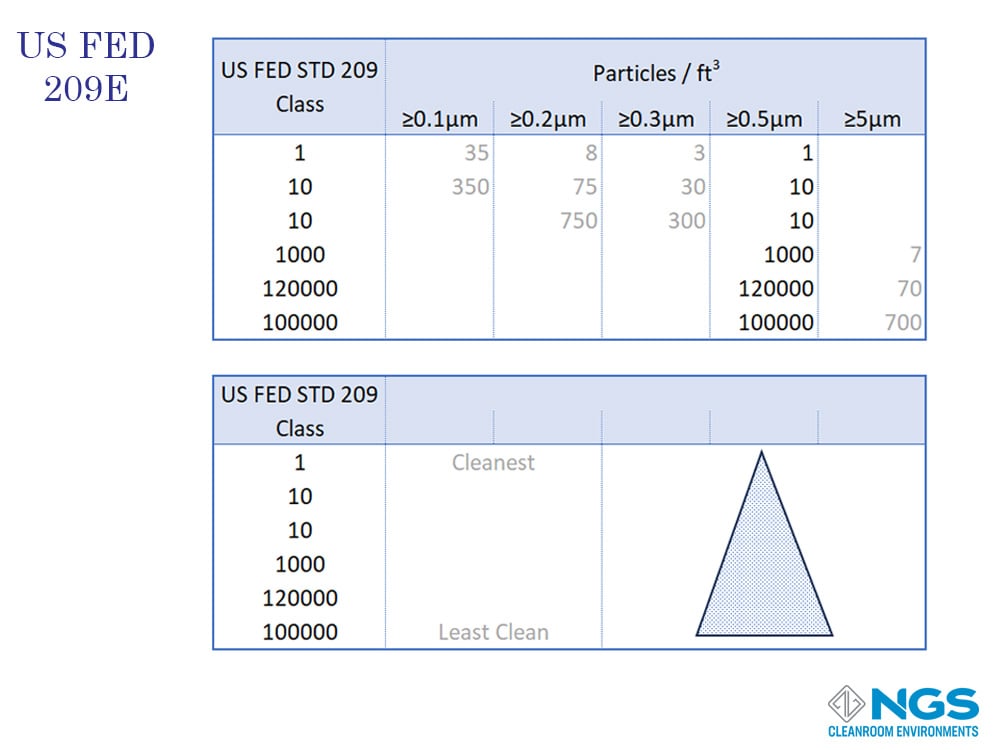
The first cleanroom standard was published in 1961 by the American Air Force and was known as Technical Manual TO 00-25-203. This standard.
The first federal standard was published in the USA in 1963 as FED STD 209A and titled ‘Cleanroom and Work Station Requirements, Controlled Environments.
Federal Standard 209 was used as the basis for the standard developed by International Organization of Standards and labelled as ISO 14644-1.
Although the Federal Standard was withdrawn in 2001 following the publication of the international standard ISO 14644-, the classifications defined in FED STD 209 are still widely used.
The cleanroom classification based on the FED standard is determined by measuring the number of particles larger than 0.5um in one cubic foot of air and then reading the class from the table.
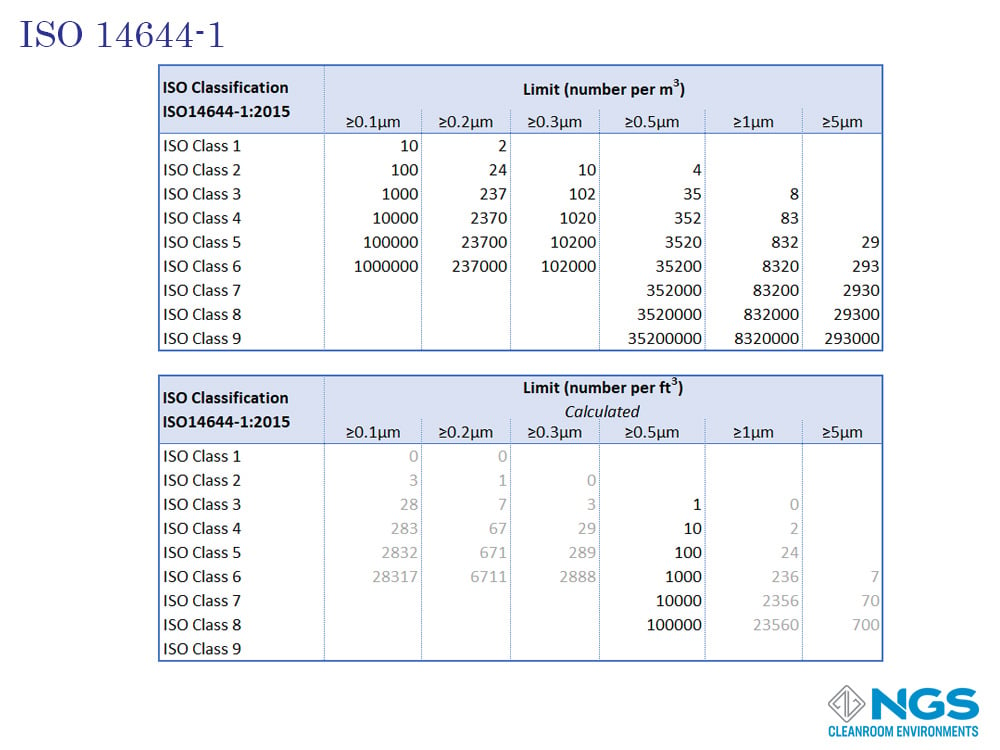
ISO 14644-1
The International Standards Organization developed 8 standards to cover the important aspects of any cleanroom system, namely classification, design, testing, operation and bio-contamination. The first part of the series ISO 14644-1 which deals with cleanroom classification was published in 1999.
The parts of the ISO 14644 standard that deal with cleanroom cleanliness are:
- Part 1: Classification of air cleanliness
- Part 2: Specifications for testing and monitoring to prove continued compliance with ISO 14644-1
- Part 3: Test Methods
- Part 4: Design, Construction, and Start-up
- Part 5: Operations
- Part 6: Vocabulary
- Part 8: Classification of airborne molecular contamination
- Part 9: Classification of surface particle cleanliness
Cleanroom Test State
When measuring the level of contamination in a cleanroom, it is important to consider the cleanroom state when the measurements were made. There are 3 main states
- At rest: All the cleanroom utilities (including HVAC) are in place and are operational but there are no personnel or operational equipment in the cleanroom. This state defines a baseline for cleanliness in the cleanroom.
- In Operation: All the cleanroom utilities (including HVAC) are in place and are operational, the equipment is functioning at the defined level and the maximum number of personnel are in the cleanroom. This is more of a ‘real world’ state for the cleanroom.
The FED and ISO standards for cleanrooms specify the contamination level in the at rest state. However, this does not really represent the ‘real world’ use and purpose of the cleanroom where personnel and working equipment may be present.
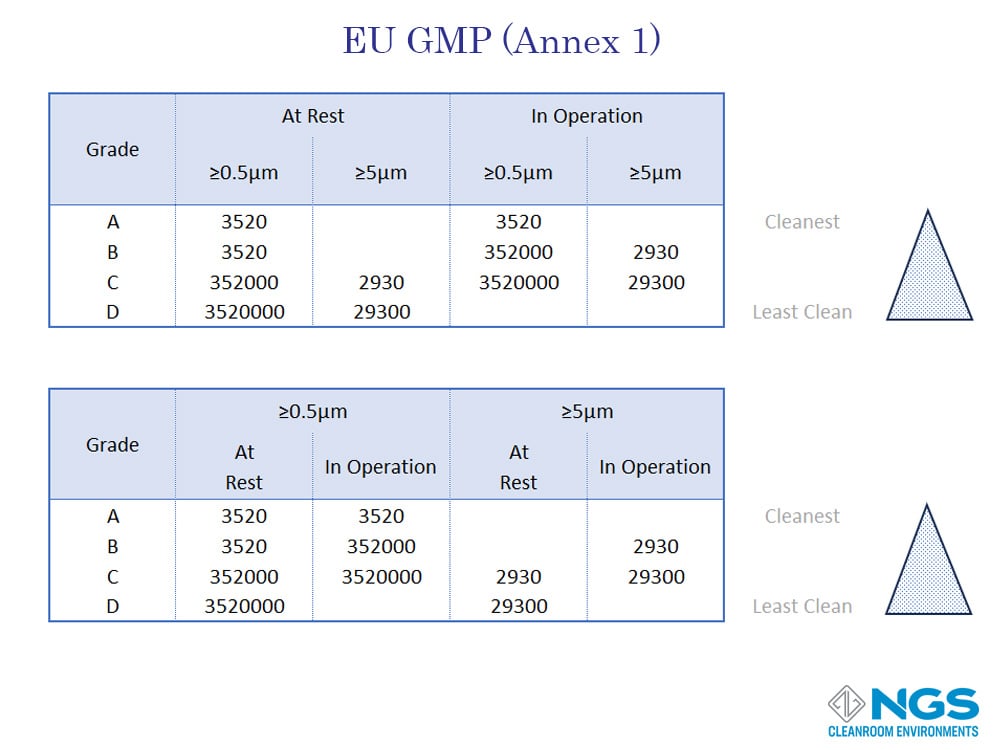
GMP Annex European Community Cleanroom Standards
The European Union Guidelines to Good Manufacturing Practices recognizes the importance of the cleanroom state and defined cleanroom Grades based on the contamination levels in a cleanroom in the at rest and in operation states. These cleanroom grades are defined in the EU GMP Annex 1 European Community Cleanroom Standards.
According to Annex 1 of the GMP standard for cleanrooms, there are four grades defined as Grade A, Grade B, Grade C, and Grade D. Each grade corresponds to specific cleanliness requirements and is associated with ISO classifications. However, the inclusion of in operation test standards makes the GMP standard more stringent than other standards
GMP standards are used in the EU by the pharmaceutical and medical industries.
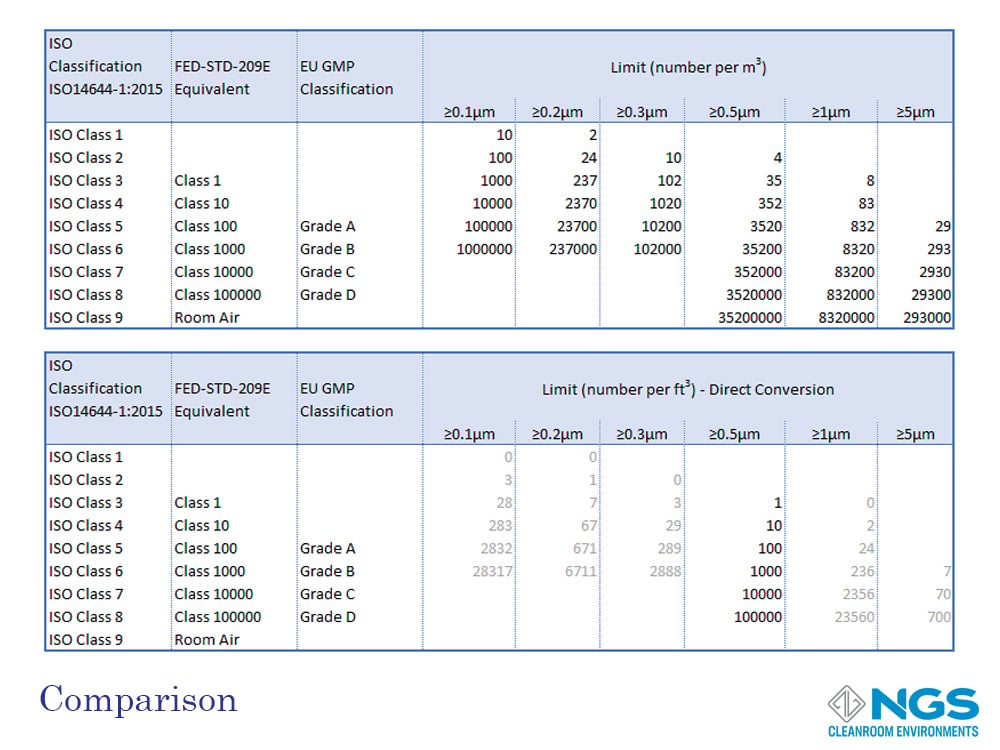
Comparing Cleanroom Standards
All cleanroom classification scales are rated according to the concentration of particulate contamination in specific sizes per unit volume. The volume may be measured in cubic meters (m3) of cubic feed (ft3). These scales range from most clean (least contamination) to least clean (highest level of contamination).
The ISO14644-1 standard goes from ISO 1 to ISO 9 with the most common range for cleanrooms being ISO 4 to ISO 8.
FED 209 defines 6 classes from Class 1 to Class 100000.
GMP has only 4 categories and these are labelled as Grade A, Grade B, Grade C and, Gade D.
A reasonable good comparison of the FED 209 and EU GMP (Annex 1) standards against the ISO 14644-1 standard for cleanrooms in the at-rest state is provided in the table shown. However, it is important to bear in mind that the GMP standard includes a separate table for the in-operation state of the cleanroom.
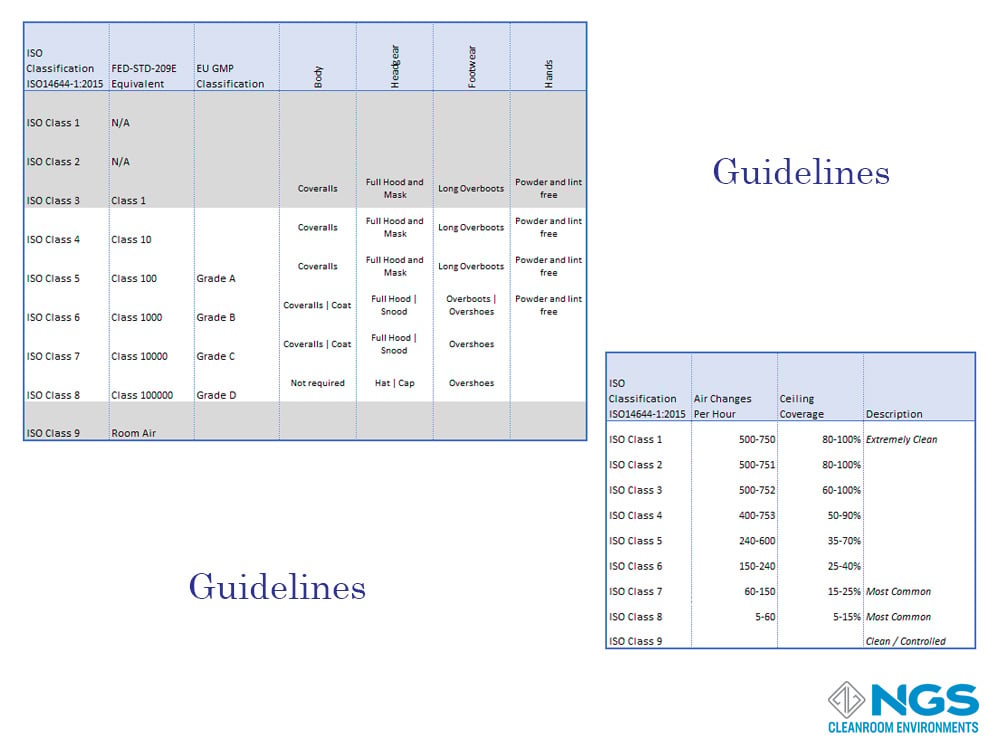
Other Guidelines
While the classification of any cleanroom in the at rest and in operation states is important, it is equally important to defines processes and procedures that are relevant to each class of cleanroom.
The processes and procedures may be specific to industry, equipment, and the application that the cleanroom is used for but there are guidelines and best-practices defined for each class of cleanroom.
These guidelines may specify the number of air changes per hour or the coverings used by personnel entering the cleanroom.
Other guidelines may include factors like temperature, humidity, lighting etc. these guidelines will be specific to the industry and the application.
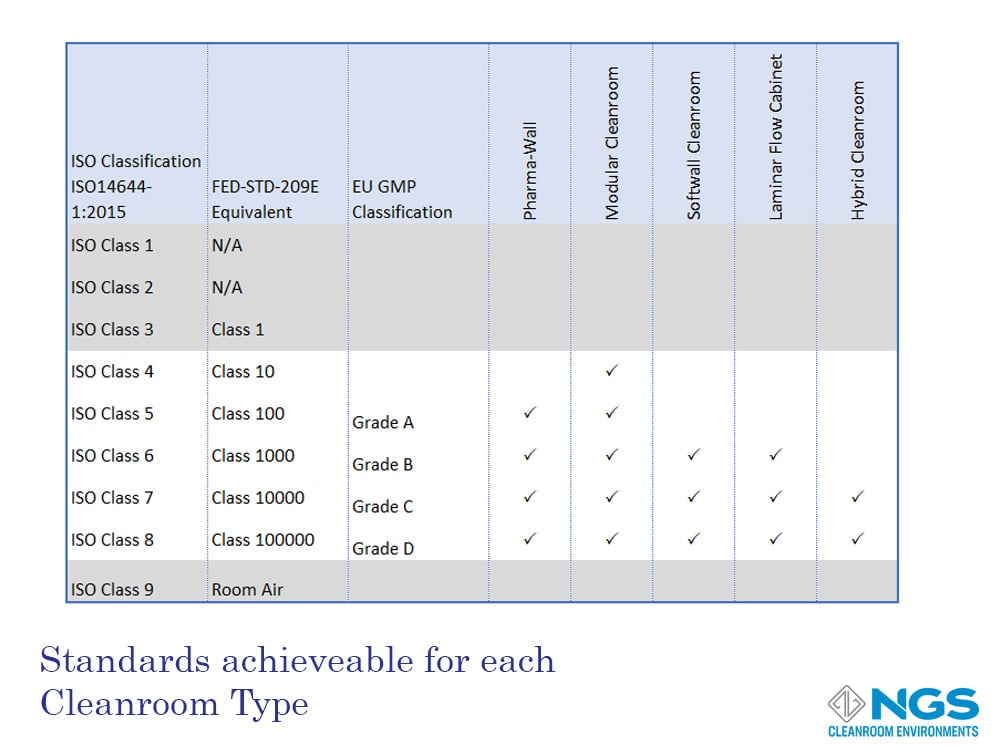
Cleanroom Types
The design of the cleanroom determines how effective the cleanroom is in preventing contamination and the level and size of the contamination entering the cleanroom.
This design includes the flooring, ceilings, walls, fixtures, HVAC and other important factors. Bearing this in mind, each cleanroom type is suited to a particular range of cleanroom classes. The type of cleanroom chosen for a particular application will therefore be determined not only by budget but also by the build constraints and the classification that needs to be achieved.
There are 5 main cleanroom types:
- Pharma-wall cleanroom;
- Modular cleanroom;
- Soft-wall cleanroom;
- Laminar flow cabinet (not strictly a cleanroom);
- Hybrid cleanroom.
The table shown lists the cleanroom types that are suited to achieve a target classification. This table considers the typical design of each type and is only intended as a guide. For example, soft-wall cleanroom designs usually do not include HVAC for air management. However, it is possible to design a soft-wall cleanroom with HVAC in order to achieve a higher classification level.